Anit-inflammation Perfecting cataract surgery
Anit-inflammation Perfecting cataract surgery
EyeWorld Supplement to EyeWorld August 2012
Inflammation
remains a significant challenge to modern cataract surgery. Left untreated,
inflammation can ruin even the most consummately performed surgery utilizing
the most advanced techniques and technology.
Experts gathered
to discuss “Ocular Anti-Inflammation: Prevention, Diagnosis, and Curative
Treatment Options” at an EyeWorld Educational Symposium held at the 2012
ASCRS•ASOA Symposium &
Congress. The
event was supported by an educational grant from ISTA Pharmaceuticals (Irvine,
Calif.).
NSAID issues 2012
“Cataract surgery
is evolving,” said Dr. Katsev. According to Dr. Katsev, patients’ standards
have been increasing in the last decade in part thanks to the increasing role
of premium IOLs in cataract surgery.
In that time, the
use of premium IOLs has increased dramatically, he said, but the promise of
great vision these IOLs make does not necessarily mean happy patients; surgeons
need to give them more. Surgeons need to take the extra step and give their
patients great vision that is also free of complications
such as cystoid macular edema (CME).
More than 20 years
ago, Dr. Katsev started using Ocufen (flurbiprofen sodium 0.3%, Allergan,
Irvine, Calif.) based on studies that showed that the drug prevented miosis in
all cases. More importantly, he said, the prevention of miosis was most
effective in patients with small pupils.
A study he
co-authored with Robert C. Drews, M.D., showed as much as a 30% decrease in pupil surface
area in small pupils when the non-steroidal anti-inflammatory drug (NSAID) was not used.1
The benefits of
using NSAIDs during cataract surgery have long been established. Inflammation
as indicated by increased anterior chamber cells and flare is known to slow
visual recovery.2,3 Topical NSAIDs have been shown to reduce postop inflammation and pain after
surgery and have typically been used in place of or in addition to
topical corticosteroids.
Other suspected
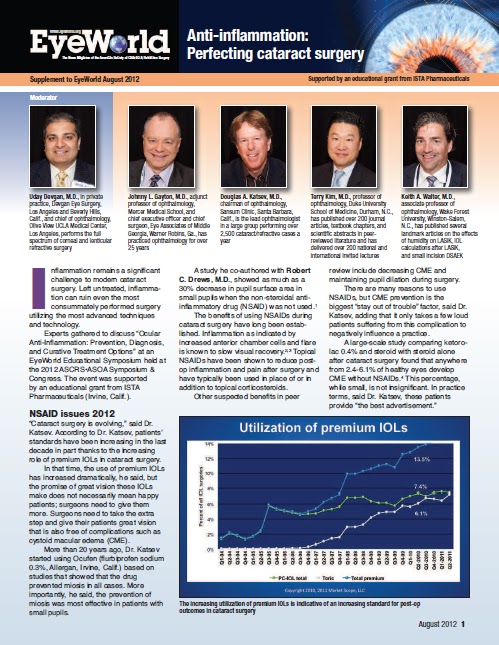
benefits in peer review include decreasing CME and maintaining pupil dilation during surgery. There are many
reasons to use NSAIDs, but CME
prevention is the biggest “stay out of trouble” factor, said Dr. Katsev,
adding that it only takes a few loud patients suffering from this complication
to negatively influence a practice.
A large-scale
study comparing ketorolac 0.4% and steroid with steroid alone after cataract
surgery found that anywhere from 2.4-6.1% of healthy eyes develop CME without NSAIDs.4 This
percentage, while small, is not insignificant. In practice terms, said Dr.
Katsev, these patients provide “the best advertisement.”
In September 2011,
pharmaceutical companies began releasing generics into the market. At the time,
generic ketorolac comprised 27.9% of NSAIDs used in cataract surgery.
Generic NSAIDs
were first introduced with generic diclofenac. Not long after the introduction
of the drug, ASCRS reported an increase in the number of corneal melts, and all NSAIDs were
pulled from the market for a period of time.
This, said Dr.
Katsev, created a fear of using NSAIDs still felt by some surgeons today.
Dr. Devgan
reported a corneal melt with a generic NSAID; since then there have been
several cases reported in the literature.
Importantly, said
Dr. Katsev, these reports show cases of corneal melt occurring with generic NSAIDs, but not
with branded NSAIDs.
There are
additional challenges regarding these generic drugs that go beyond the
clinical, said Dr. Katsev. In June 2011, the Supreme Court came out with a
ruling that essentially freed generics manufacturers from the responsibilities
for updating their labeling held over branded product manufacturers. Branded
product manufacturers need to protect their names; with generics, they are not
beholden to protecting that name.
Dr. Katsev’s first
encounter with low quality generics was
in a patient with diabetes in whom he had implanted bilateral premium IOLs. He
initially started the patient on Bromday (bromfenac sodium, ISTA
Pharmaceuticals). However, at the pharmacy, the patient was instead given
generic ketorolac.
The patient did
not return until he had CME. By going back on Bromday, his vision was restored
to 20/25. According to the patient, he needed to
stop using the generic ketorolac due to discomfort and did not use
any NSAID until his follow-up consult, by which time it was almost too late.
“We
as surgeons need to be vigilant about the quality of the drugs we use in our
patients,” said Dr.
Katsev. With generics, he said, the medication is
supposed to be the same, but the bottle, the pH, all of these things are
different and may have an effect on the quality of the drug.
“There may be
great generics, but there are also bad generics, and it is up to us to keep
watch over these drugs to maintain a high standard for cataract surgery in our
patients.”
References
1. Drews RC,
Katsev DA. Ocufen and pupillary dilation during cataract surgery. J Cataract
Refract Surg. 1989;15(4):445-448.
2. Kim SJ, Flach
AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv
Ophthalmol. 2010;55:108-133.
3. O’Brien TP.
Emerging guidelines for use of NSAID therapy to optimize cataract surgery
patient care. Curr Med Res Opin. 2005;21:1131-1137.
4. Wittpenn JR,
Silverstein S, Heier J, et al. Acular LS for Cystoid Macular Edema (ACME) Study
Group. A randomized, masked comparison of topical ketorolac 0.4% plus steroid
vs. steroid alone in low-risk cataract surgery patients. Am J Ophthalmol.
2008;146:554-560.
Corneal melts/toxicity:
Is there still an issue?
The reported
complications following the use of NSAIDs in cataract surgery vary in severity,
according to Dr. Kim. These cases range from things as innocuous as superficial
punctate keratitis, to stromal infiltrates, immune rings, and persistent epithelial
defects, to the most dreaded complication: corneal melts.
Dr. Kim said this
was the major issue back in 1999, when the ASCRS survey came out looking at
bservations of corneal melt after routine anterior segment surgery.
The survey
pinpointed the use of topical generic diclofenac, which was voluntarily
identified and recalled by the manufacturer. There have been many similar
reports with other NSAIDs, said Dr. Kim.
“There is still a
lot of fear among cataract surgeons when using NSAIDs during cataract surgery,”
he said.
The
deleterious effect of NSAIDs on the cornea is thought to be related to a group
of proteases or ollagenolytic enzymes called matrix metalloproteinases (MMPs).
The MMP family is
very large; to date, studies have identified 20 MMPs expressed in humans. These
enzymes have multiple functions and are known to degrade extracellular matrix
(ECM) and enhance cell-cell, cell-matrix communications. Rarely detected in
normal tissues, MMPs are typically expressed in tissues undergoing rapid
turnover, such as during tumor breakout, normal bone and joint formation, and
wound healing.
In the eye, MMPs
are involved in many physiologic and pathophysiologic processes, said Dr. Kim.
These include disease conditions like macular degeneration and diabetic
retinopathy and processes such as IOP regulation.
In the cornea
specifically, MMPs have been detected in corneal ulcers, keratoconus, and after
PRK surgery.
NSAIDs have been potentially linked to corneal melts
through the upregulation of MMPs,
resulting in an imbalance between ECM deposition and degradation. NSAIDs may
cause excessive MMP expression, and various MMPs have been found in
NSAID-related melts.
Clinically,
topical NSAIDs decrease normal corneal sensation1,2 and can affect corneal
epithelial healing.3,4
To avoid
complications, NSAIDs should be used properly. “I believe it is very important
to follow the label dosing,” said Dr. Kim. “If you look at case series that are
reported in the literature, complications like corneal melt usually occur when NSAIDs are not dosed
properly.”
With improper
dosing, he said, NSAIDs can cause complications within 2 hours of use. “I would
also recommend avoiding
longterm use of topical NSAIDs,” he added.
It is
important to also examine patient characteristics, looking at risks for corneal
melt.
Patients with epithelial keratopathy or severe dry eyes, bacterial
keratitis, herpes simplex or zoster keratitis, ocular surface disease,
concurrent topical steroids, or systemic disorders predisposing them
to corneal melts such as diabetes
and rheumatoid arthritis
need to be followed more closely. In addition, “Always make sure there is no active infection in patients undergoing
topical NSAID therapy,” said Dr. Kim.
Established safety
profiles are a very important consideration, he added; for instance, there have
been no reports of corneal melt in clinical trials using Bromday. The
once-daily dosing of Bromday, which has been reportedly used for up to 3 or 4
months and longer, is particularly relevant for patients at high risk for
corneal melt, but also an important consideration for the routine patient.
Complications with NSAIDs are fairly uncommon, but it’s
important to pay attention to high-risk patients.
“Multiple factors
are involved in corneal melt and NSAID toxicity,” said Dr. Kim. “We need
further studies to elucidate the mechanisms that are involved and the specific
role of MMPs.”
References
1. Szerenyi K,
Sorken K, Garbus JJ, et al. Decrease in normal human corneal sensitivity with
topical diclofenac sodium. Am J Ophthalmol. 1994;118:312-315.
2. Sun R, Gimbel
HV. Effects of topical ketorolac and diclofenac on normal corneal sensation. J
Refract Surg. 1997 Mar-Apr; 13:158-161.
3. Hersh PS, Rice
BA, Baer JC, et al. Topical nonsteroidal agents and corneal wound healing. Arch
Ophthalmol. 1990;108:577-583.
4. Assouline M,
Renard G, Arne JL, et al. A prospective randomized trial of topical soluble
0.1% indomethacin versus 0.1% diclofenac versus placebo for the control of pain
following excimer laser photorefractive keratectomy. Ophthalmic Surg Lasers.
1998;29:365-374.
My routine Rx: Insights on
compliance and dosing
The medical
literature strongly indicates that non-compliance ranges up to 50% of various
dosing regimens.1 Significantly, compliance worsens with increasing age.2
“There are three
signs of getting old,” said Dr. Gayton. “One is losing your memory, and I can’t
remember what the other two are. The truth is, you do tend to forget. That’s
just a fact.”
According to Dr.
Gayton, as shown by previous studies, one thing that can improve compliance is
less frequent dosing.3,4
In a survey of Dr.
Gayton’s own patients comparing Pred Forte (prednisolone, Allergan) and Durezol
(difluprednate, Alcon, Fort Worth, Texas), most of them said they preferred
Durezol because of its
less frequent
dosing.
There are many
factors that correlate with compliance, including dosing regimen, patient
lifestyle routines, use of other medication, and side effects. “We are used to
hearing that if a medication doesn’t burn or taste bad, it isn’t good for you,”
said Dr. Gayton. “The truth is, patients don’t like a drop that burns, and they
will tend not to use it.”
Bromday, he noted,
is a comfortable drop to use. “It isn’t sticky and it does not burn.”
The number of
drops a patient has to use each day is another factor; studies have shown that
drops used just once or twice a day may improve compliance over more frequent
dosing.3 Meanwhile, on-compliance in
glaucoma patients who tend to need two or more instillations of various drops
per day has been noted to go up to 59%.5
Looking into
compliance in post-op cataract surgery patients revealed that all were
non-compliant at some point in terms of total dose, time intervals, and
premature discontinuation of therapy.6
Different
therapies have different advantages and disadvantages.
Studies have shown
that use of steroids alone
for long-term treatment has a greater risk of IOP rise and is attended by a
definite increase in macular thickening or CME.7,8
On the other hand,
steroids reduce the amount of arachidonic acid available for the cox enzymes to
convert prostaglandins, and it is reasonable to believe, said Dr. Gayton, that
using NSAIDs alone may lose some efficacy by missing the lipoxygenase
pathway.
“Combined therapy can be very efficacious in safely
controlling acute and chronic inflammation,” he said. “And because we know that the peak incidence of CME is 4-6 weeks,9
it makes sense to use therapy for at least that amount of time.”
It’s very
important to use your NSAIDs long enough especially in high-risk cases,he said.
Dr. Gayton
switched to using Bromday (at the time marketed as Xibrom) on the basis of
results from rabbit and rat studies that demonstrated that the drug remained at table concentrations in ocular tissues for
more than 24 hours. These studies, he said, showed that the drug could
be safely used with once-a-day dosing.10,11
In Dr. Gayton’s
practice, he begins instillation of drops including NSAIDs and antibiotics
several days before surgery.
On the question of
using generics or branded drugs, “we strongly prefer Bromday,” he said. “In fact, we tell our patients that if they
have to choose between all of the different medications and can only pick one
branded drug, we ask them to make that branded drug the NSAID. The NSAID
carries the greatest
risk and the
greatest benefit.”
In Dr. Gayton’s
clinic, they use antibiotics three times a day 6 days before surgery and Bromday 7 days before surgery;
the antibiotics are continued out to 14 days because of research that’s shown
the peak incidence of endophthalmitis is 10-14 days.
“We’ll use NSAIDs at least 6 weeks if possible,” he said. “That’s one of the
big advantages of a drop that’s used one time a day because people are much
more likely to comply.”
Dr. Gayton
combines NSAID therapy with a steroid, although in non-diabetic patients, the steroid is discontinued much earlier, stopping anywhere from 2-3 weeks. “Patients who are at high
risk will continue the steroid for a significantly longer period of time, maybe
going out as long as 6
weeks; [we
recognize] that we have to follow those patients more closely because of the
increased risk of sequela such as ocular surface issues,” he added.
To help with
regard to compliance, Dr. Gayton suggested keeping in mind what he calls the Cs: “You need to have
good corneal penetration,
you need excellent cox enzyme binding, you need rapid and total clearance of inflammation,
and you need to have a drop that’s comfortable and convenient to use.”
References
1. Koberlein J,
Kothe AC, Schaffert C. Determinants of patient compliance in allergic
rhinoconjunctivitis. Curr Opin Allergy Clin Immunol. 2011;11(3):192-199.
2. Hoy SM, Keam
SJ, Keating GM. Travoprost/Timolol. Drugs Aging. 2006;23(7):587-597.
3. Ikeda H, Sato
M, Tsukamoto H, et al. Evaluation and multivariate statistical analysis of
factors influencing patient adherence to ophthalmic solutions. Yakugaku Zasshi.
2001;121(11):799-806.
4. Cremer J.
Medicine Partnerships. Heart. 2003;89 (suppl II):ii19-ii21.
5. Patel SC,
Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic
Surg. 1995;26(3):233-236.
6. Hermann MM,
Ustundag C, Diestelhorst M. Compliance with topical therapy after cataract
surgery using a new microprocessor-controlled eye drop monitor. Invest Ophthalmol
Vis Sci. 2005;46. E-abstract 3832.
7. Raizman M.
Macular edema after cataract surgery. Presented at the Royal Hawaiian Eye
Meeting; Jan 24-29, 1999; Waikoloa, Hawaii.
8. Wittpenn JR,
Silverstein S, Heier J, et al. Acular LS for Cystoid Macular Edema (ACME) Study
Group. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs. steroid
alone in low-risk cataract surgery patients. Am J Ophthalmol. 2008;146:554-560.
9. Asano S, Miyake K,
Ota I, et al. Reducing angiographic cystoid macular edema and blood-aqueous
barrier disruption after small-incision phacoemulsification and foldable
intraocular lens implantation: Multicenter prospective randomized comparison of
topical diclofenac 0.1% and betamethasone 0.1%. J Cataract Refract Surg. 2008;34(1):57-63.
10. Baklayan GA,
Patterson HM, Song CK, et al. 24-hour evaluation of the ocular distribution of
(14) c-labeled bromfenac following topical instillation into the eyes of New
Zealand white rabbits. J Ocul Pharmacol Ther. 2008;24:392-398.
11. Data on file, ISTA
Pharmaceuticals.
NSAID alone vs.
NSAID + steroid
Are steroids really
necessary in routine cataract surgery? asked Dr. Walter.
In October 2010, he
said that steroids stopped being a routine part of his cataract kits. “At the
time, I was frustrated by how ineffective generic steroids were, and I was hesitant
to write a prescription for yet
another eye drop,” he
said.
Since then, Dr. Walter
said that he only uses Bromday. He begins therapy 2 days before surgery and has
the patient use it until the bottle runs out, an offlabel— but effective, in
his experience— use of the drug
“I believe I could always
add a steroid if I need to,” he said. Dr. Walter used this protocol on all
hispatients, and he recently published data from his experience in U.S.
Ophthalmic Review.1
In this study, Dr.
Walter and his colleagues looked retrospectively at 200 consecutive eyes in
which he had used Pred
Forte 1% four times a day for about 5 weeks. About 12% of those patients
received Bromday as well.
They also looked at
200 consecutive eyes where he only used Bromday; none of those patients had
supplemental steroids.
Dr. Walter performed
surgery on most of the eyes, leaving about 20% to two different fellows. They
included “all comers”—their patients with diabetes, floppy iris, and hard
nuclei. They looked at pain, inflammation, BCVA, CME, and post-op IOP as
endpoints. They had follow-up in 178 eyes with Pred Forte and 169 eyes with
Bromday.
The vision between the
two groups of patients was about the same, with BCVA 1 month post-op at 20/27.2
and 20/26.6, respectively. At 2 weeks post-op, inflammation was seen in 16 (8%)
Pred Forte group eyes and 24 (12%) Bromday eyes. Of note, Dr. Walter said that
two out of the 24 eyes in the Bromday group had retained nuclear fragments.
They had 44 patients
respond to a survey. These patients rated painintraoperatively and post-op at
around 1 on a scale from 0 to 10, indicating very low pain either intra- or
post-op. Only 2 (1%) eyes in the Pred Forte group and 1 (0.5%) in the Bromday
group had CME, detected clinically and confirmed with OCT.
IOP elevation proved
to be an interesting endpoint, said Dr. Walter. The total number of eyes with
IOP elevations of 5 mm Hg or
higher from pre-op baseline was 16 (8%) in the Pred Forte group and 7 (3.5%) in
the Bromday group (p=0.08). Note that the eyes with elevated IOP in the
Bromday group included the two eyes with retained nuclear fragments.
Looking at eyes with a
history of glaucoma, 8 out of 25 (32%) in the Pred Forte group and none out of 17 in the Bromday group had IOP elevation.
Remember that these
are all comers, including patients with floppy irises, diabetes, and dense
nuclei. “Why haven’t we needed to add a steroid in any of my last 900 cases
[including the 200 who were part of this study]?” wondered Dr. Walter.
“I think it comes down
to this: It may be that we are so engrained to use a steroid that it’s
impossible to consider doing otherwise in these cases,” he said.
It must also have to
do with the advances in pharmaceutical technology. “NSAIDs have gotten better
and better with each additional newcomer on the block,” said Dr. Walter.
“Bromday is a better NSAID than any we’ve had before.
“It’s made my life
simpler because now my patients have a much simpler drop table,” he continued.
“Compliant patients have had no CME yet, and we have not had any corneal
complications.”
Reference
1. Walter K, Estes A,
Watson S, Ellingboe M. Management of Ocular Inflammation following Routine
Cataract Surgery—Topical Corticosteroid (Prednisolone) versus Topical
Non-steroidal (Bromfenac). US Ophthalmic Review, 2011;4(2):97-100.
2. Duong HQ, Westfield
KC, Singleton IC. Comparing Three Post-Op Regiments for Management of
Inflammation Post Uncomplicated Cataract Surgery. “Are Steroids Really
Necessary?” J Clinic Experiment Ophthalmol 2011; 2:6.
3. Cable M. Clinical
Outcomes of Bromfenac Ophthalmic Solution 0.09% QD vs. Nepafenac 0.1% TID for
Treatment of Ocular Inflammation Associated with Ocular Surgery. Presented at
ARVO 2012
Contact information
Devgan:
devgan@gmail.com
Gayton:
JLGayton@aol.com
Katsev: katsev@aol.com
Kim:
terry.kim@duke.edu
Walter:
kwalter@wfubmc.edu





留言
張貼留言