Study finds new possible treatment for pterygium
July 2011‧ Eye World
Study finds new possible treatment for pterygium
by Matt Young EyeWorld Contributing Editor
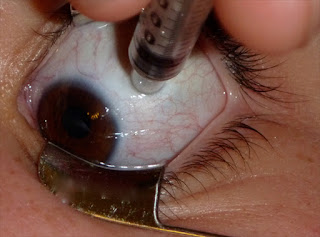 |
| A patient is injected with bevacizumab. A recent study indicates that these injections may be a possible treatment for pterygium Source: María H. Berrocal, M.D. |
In the future, it may be possible that the cure for pterygium isn't total surgical excision. A new report hints that some concoction of bevacizumab (Avastin, Genentech, South San Francisco, Calif.) could be injected and reduce the size of pterygium.
The research, published online in Cornea in September 2010, found that intralesional bevacizumab injection reduces the size of pterygium and is well tolerated. However, there was no clinically significant impact on the reduction in pterygium size. The fact that bevacizumab worked to reduce pterygium size still holds promise.
Current methods to excise pterygium surgically and prevent recurrence have side effects, including punctate epitheliopathy, bacterial superinfection, delayed onset sclera melting, and IOP rise.
Given that bevacizumab was both effective in reducing pterygium size and safe, it may just be a matter of tweaking the number of injections to cause an even greater size reduction.
"Repeated injection of bevacizumab (to decrease the pterygium size more efficiently) or its adjuvant role before or during surgical removal of pterygium may help to address its clinical significance in future studies," according to the report, co-authored by Mehrdad Mohammadpour, M.D., Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Iran.
A decrease in size
Pterygium was analyzed in 17 patients (14 with primary pterygium and three with recurrent pterygium). Patients received intralesional injections of bevacizumab (2.5 mg/0.1 mL). Injection occurred at the base of the pterygium with a 25-gauge needle. All patients received a single injection, except two primary cases and one recurrent case, which received a second injection.
Prior to injection, mean lesion size was 17.2%+/–4.3% of the corneal surface. Lesions reduced to 15.1%+/–4.3% at 1 week after injection, 13.4%+/–4.0% at 1 month, and 14.1%+/–4.4% at 3 months. Lesion size was determined with the help of digital photographs and image analysis software.
The mean percentage decrease was 3.97%+/–3.84% in primary pterygium cases. The decrease in size before injection and after at all time periods was statistically significant.
Notably, recurrent pterygium cases did experience lesion progression (by 1.11%+/–0.450%). Also, visual acuity did not change significantly in any patient after the injections.
"It is postulated that for control of corneal neovascularization, higher doses of bevacizumab than the classic 2.5-mg dosage given in intravitreal injections may be needed for subconjunctival injections," Dr. Mohammadpour reported. Pterygia "are known to depend on neovascularization," Dr. Mohammadpour noted.
Mechanism of action
Bevacizumab likely reduces the size of pterygium due to its anti-VEGF action.
"Several active angiogenic and epithelial growth factors such as basic fibroblast growth factor, heparin-binding epidermal growth factor, connective tissue growth factor, and vascular endothelial growth factor (VEGF) have been shown to be significantly increased in pterygium, suggesting that growth factors may be involved directly or indirectly in its pathogenesis," Dr. Mohammadpour reported. "However, the most prominent of these factors is VEGF, which is the main target of many current antiangiogenic therapies. Bevacizumab is a monoclonal antibody to VEGF A that binds to both VEGF receptors VEGFR2 and VEGFR1 and inhibits both receptors."
Already, research has suggested bevacizumab is effective for the treatment of corneal neovascularization, Dr. Mohammadpour noted.
Literature about the impact of bevacizumab on pterygium, however, has been mixed. One study found anti-VEGF treatment controlled pterygium inflammation, while another found bevacizumab had no impact on corneal vessel density in recurrent pterygia. In another study, topical bevacizumab was used to treat impending recurrent pterygium with success.
"The promising point of view is that in these studies, there was no reported adverse effect for bevacizumab," Dr. Mohammadpour concluded. "The important question is to quantify the therapeutic concentrations of such antibodies in the cornea."
Francis S. Mah, M.D., co-medical director, Charles T. Campbell Ophthalmic Microbiology Laboratory, University of Pittsburgh School of Medicine, said that other research institutions have looked at anti-VEGF treatment for pterygium removal, calling it a "hot topic" for surgeons.
"We don't know how to best apply the medication," Dr. Mah said. "We also don't know the best dosage."
Dr. Mah believes more research needs to be done to study the short-term effects of drug use, such as potential toxicity. "This is a hopeful avenue with a lot of unanswered questions," Dr. Mah said.
Editors' note: Dr. Mah has no financial interests related to his comments. Dr. Mohammadpour has no financial interests related to this study.
Contact information
Mah: 412-647-2211, mahfs@upmc.edu
Mohammadpour: mohammadpour@yahoo.com


留言
張貼留言